Well-Defined Amylose Acetate-graft-polylactide Graft Polymers as Compatibilizers for Renewable Polymer Blends
Authors:
Jeffrey E. Thompson; Isabela T. Coutinho; Nicholas F. Pietra; Louis A. Madsen; Robert B. Moore; Kevin J. Edgar
Affiliation:
Macromolecules Innovation Institute and Department of Chemistry, Virginia Tech, Blacksburg, Virginia 24061, United States
Description:
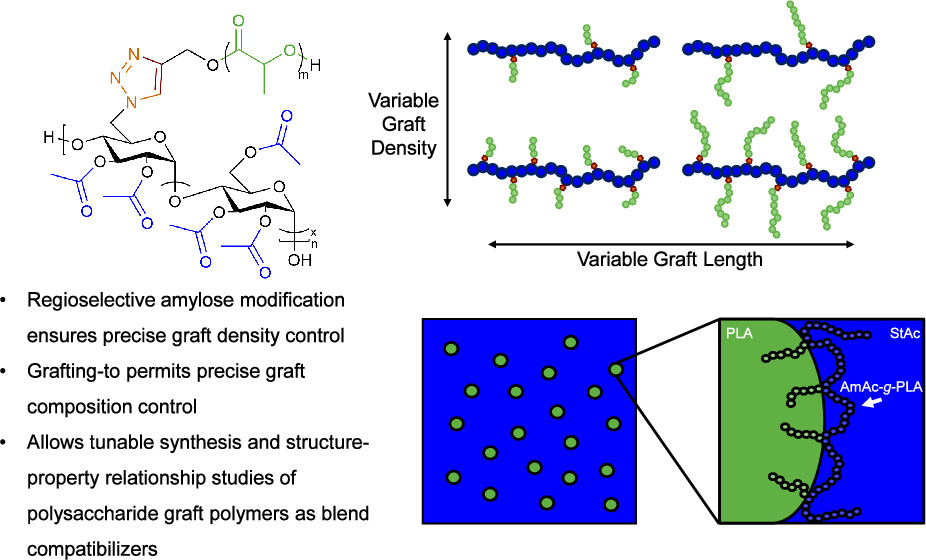
Regioselectively substituted amylose acetate-graft-polylactide (AmAc-g-PLA) graft polymers were synthesized via grafting-to “click” reaction between C6-azide functionalized AmAc and alkyne-terminated PLA. Alkyne-terminated PLA synthesized through organocatalytic ring-opening polymerization (ROP) permitted control over graft degree of polymerization (DP) and stereochemistry, while azide functionalized AmAc with tailorable C6-azide degree of substitution (DS) allowed graft density control. This describes the first synthesis of polysaccharide-based graft polymers with exclusive C6 grafting and controllable topology. Thermal analysis indicates that glass transition temperatures (Tg) of AmAc and PLA segments are affected after grafting-to coupling, with poly(l-lactide) (PLLA) grafts maintaining crystallizability. AmAc-graft-poly(d,l-lactide) (PDLLA) graft polymers were effective compatibilizers for immiscible blends of starch acetate (StAc) and PDLLA as evidenced by small-angle laser light scattering (SALLS) and phase contrast optical microscopy (PCOM). This method permits the determination of structure–property relationships with regard to the effect of graft polymer topology on blend compatibilization, which will be invaluable in designing compatibilized biobased polymer blends as sustainable materials.
Publications:
- Jeffrey E. Thompson; Isabela T. Coutinho; Nicholas F. Pietra; Louis A. Madsen; Robert B. Moore; Kevin J. Edgar; Well-Defined Amylose Acetate-graft-polylactide Graft Polymers as Compatibilizers for Renewable Polymer Blends; Biomacromolecules, 2025
Tags:
Nuclear magnetic resonance spectroscopy PolymersRelated Chronicles:
No related chronicles available
Files:
No related files available