Mutant gltS alleles enable a Vibrio fischeri D-glutamate auxotroph to grow with lower requirements for exogenous D-glutamate
Authors:
Macey Coppinger1,2, Richard F. Helm3, Liu Yang4, Edward G. Ruby4, David L. Popham5, Eric V. Stabb2
Affiliation:
Description:
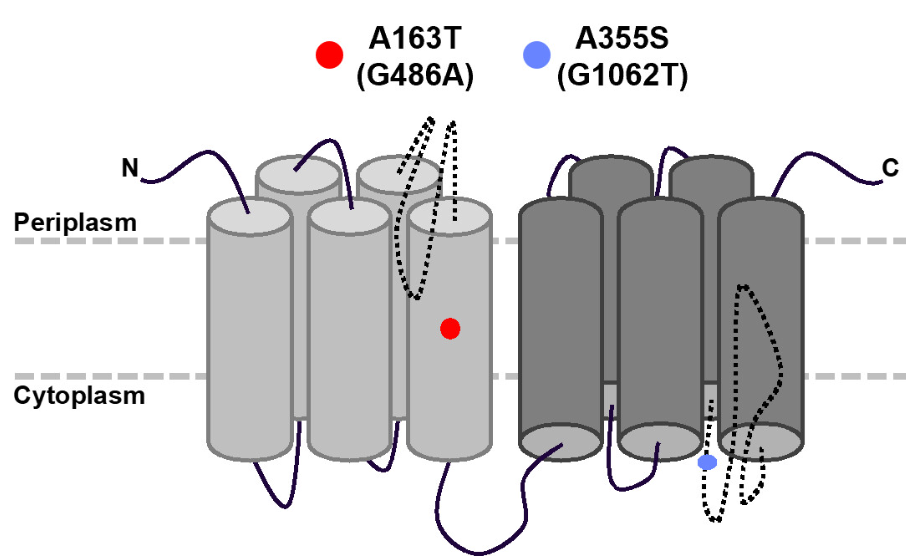
D-glu is a key component of peptidoglycan (PG) and is essential for growth in most bacteria. To assess constraints on PG evolution and bacterial requirements for D-glu, we sought to artificially evolve PG biosynthesis, leading to either replacement of D-glu in the PG peptide or alternative pathways to D-glu incorporation. We previously found that suppression of D-glu auxotrophy in a murI racD mutant of Vibrio fischeri grown on lysogeny broth salts (LBS) medium was rare but could be accomplished by mutation of bsrF, with restoration of wild-type PG structure. Here, we selected nine additional prototrophic suppressors of the same murI racD mutant from 1010 colony-forming units (CFU) plated on LBS supplemented with ~2.7 mM D-gln. Each suppressor had a mutation in gltS, which encodes a putative sodium:glutamate symporter. Increased copy numbers of mutant gltS alleles enabled growth on unsupplemented LBS and resulted in PG containing D-glu. Examination of media components suggests that D-gln supplementation had inadvertently added ~14 μM D-glu, and that LBS itself contains ~1.4 μM D-glu. The mutations in gltS enabled growth with similarly low D-glu concentrations, but also increased sensitivity to homocysteic acid, suggesting more promiscuous transport. Surprisingly, we discovered that expression of mutant gltS in the auxotroph leads to incorporation of lysine into PG, in addition to canonical D-glu. When seawater is supplemented with D-glu, this V. fischeri mutant still colonized Euprymna scolopes and triggered PG-induced morphogenesis. Our results shed light on glutamate transport, highlight trade-offs in GltS structure and function, and reveal an unusual PG modification.
Publications:
- Macey Coppinger, Richard F. Helm, Liu Yang, Edward G. Ruby, David L. Popham, Eric V. Stabb; Mutant gltS alleles enable a Vibrio fischeri D-glutamate auxotroph to grow with lower requirements for exogenous D-glutamate; Microbiology Spectrum, 2025
Tags:
Amino acids Mass spectrometryRelated Chronicles:
No related chronicles available
Files:
No related files available