Thermodynamics of calcium binding to heparin: Implications of solvation and water structuring for polysaccharide biofunctions
Authors:
Brenna M. Knight, Connor M. B. Gallagher, Michael D. Schulz, Kevin J. Edgar, Caylyn D. McNaul, Christina A. McCutchin, and Patricia M. Dove
Affiliation:
Description:
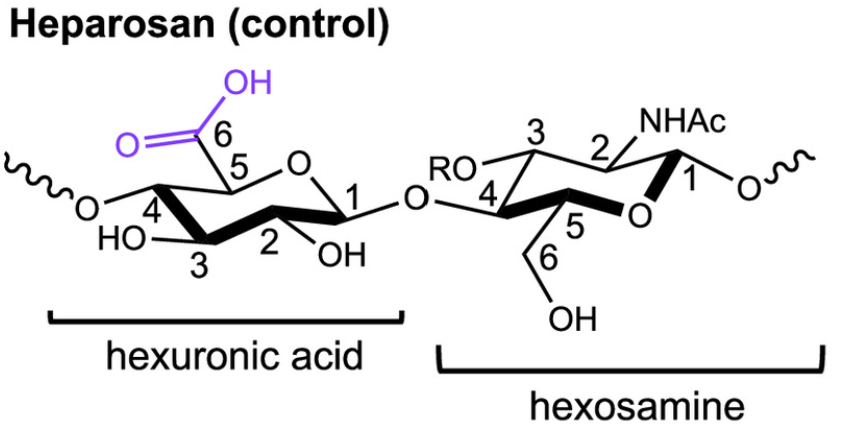
Heparan sulfates are found in all animal tissues and have essential roles in living systems. This family of biomacromolecules modulates binding to calcium ions (Ca2+) in low free energy reactions that influence biochemical processes from cell signaling and anticoagulant efficacy to biomineralization. Despite their ubiquity, the thermodynamic basis for how heparans and similarly functionalized biomolecules regulate Ca2+ interactions is not yet established. Using heparosan (Control) and heparins with different positions of sulfate groups, we quantify how SO3− and COO− content and SO3− position modulate Ca2+ binding by isothermal titration calorimetry. The free energy of all heparin-Ca2+ interactions (ΔGrxn) is dominated by entropic contributions due to favorable water release from polar, hydrophilic groups. Heparin with both sulfate esters (O-SO3−) and sulfamides (N-SO3−) has the strongest binding to Ca2+ compared to heparosan and to heparin with only O-SO3− groups (~3X). By linking Ca2+ binding thermodynamics to measurements of the interfacial energy for calcite (CaCO3) crystallization onto polysaccharides, we show molecule-specific differences in nucleation rate can be explained by differences in water structuring during Ca2+ interactions. A large entropic term (-TΔSrxn) upon Ca2+–polysaccharide binding correlates with high interfacial energy to CaCO3 nucleation. Combining our measurements with literature values indicates many Ca2+–polysaccharide interactions have a shared thermodynamic signature. The resulting enthalpy–entropy compensation relationship suggests these interactions are generally dominated by water restructuring involving few conformational changes, distinct from Ca2+–protein binding. Our findings quantify the thermodynamic origins of heparin-specific interactions with Ca2+ and demonstrate the contributions of solvation and functional group position during biomacromolecule-mediated ion regulation.
Publications:
- Brenna M. Knight, Connor M. B. Gallagher, Michael D. Schulz, Kevin J. Edgar, Caylyn D. McNaul, Christina A. McCutchin, and Patricia M. Dove; Thermodynamics of calcium binding to heparin: Implications of solvation and water structuring for polysaccharide biofunctions; Proceedings of the National Academy of Sciences, 2025
Tags:
Carbohydrates Heparin Nuclear magnetic resonance spectroscopyRelated Chronicles:
Files:
No related files available