Efficient, Regioselective Design of Mixed Cellulose Esters and Macroinitiators
Authors:
Jeffrey E. Thompson*, Kevin J. Edgar
Affiliation:
Macromolecules Innovation Institute, Virginia Tech, Blacksburg, Virginia 24061, United States
Department of Sustainable Biomaterials, Virginia Tech, Blacksburg, Virginia 24061, United States
Description:
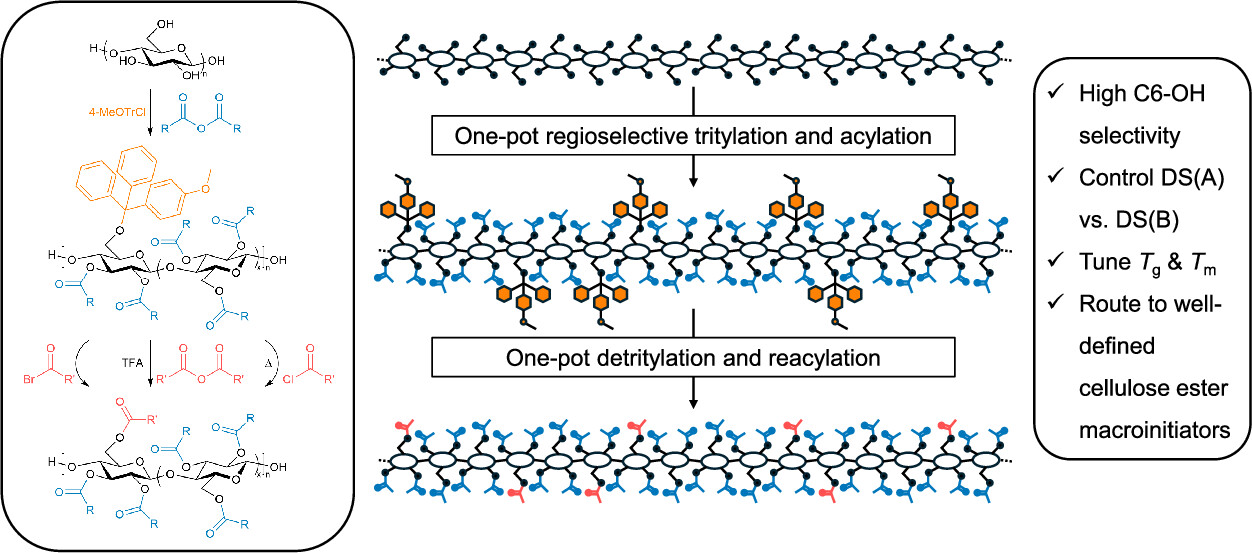
Mixed cellulose esters and macroinitiators with high C6-OH regioselectivity were synthesized by employing a tritylation protecting strategy, using sequential one-pot protection/acylation and deprotection/reacylation transformations, reducing the number of overall discrete steps and product isolations by half. This method produced organosoluble 2,3-di-O-acyl-6-O-(4-monomethoxytrityl) (2,3A-6MeOTr) cellulose intermediates, simplifying the generation of regioselectively substituted cellulose esters with controllable degree of substitution (DS) and substitution position. Treatment of 2,3A-6MeOTr celluloses with carboxylic acid anhydrides and trifluoroacetic acid (TFA) or acyl halides resulted in one-pot detritylation and reacylation of protected C6-OH groups without acyl migration or significant backbone degradation. Thermal analysis indicated that both DS and C6 compositions affected the glass transition temperature (Tg) of mixed cellulose esters, highlighting the value of this method for structure–property relationship elucidation. Notably, mixed 2,3-di-O-A-6-O-B (2,3A-6B) cellulose esters with DS(A) > 2.69 were semicrystalline. This method permits the facile generation of regioselectively substituted 2,3A-6B cellulose esters, including well-defined macroinitiators for controlled radical polymerization.
Publications:
- Jeffrey E. Thompson*, Kevin J. Edgar; Efficient, Regioselective Design of Mixed Cellulose Esters and Macroinitiators; Biomacromolecules, 2026
Tags:
Cellulose Nuclear magnetic resonance spectroscopyRelated Chronicles:
No related chronicles available
Files:
No related files available